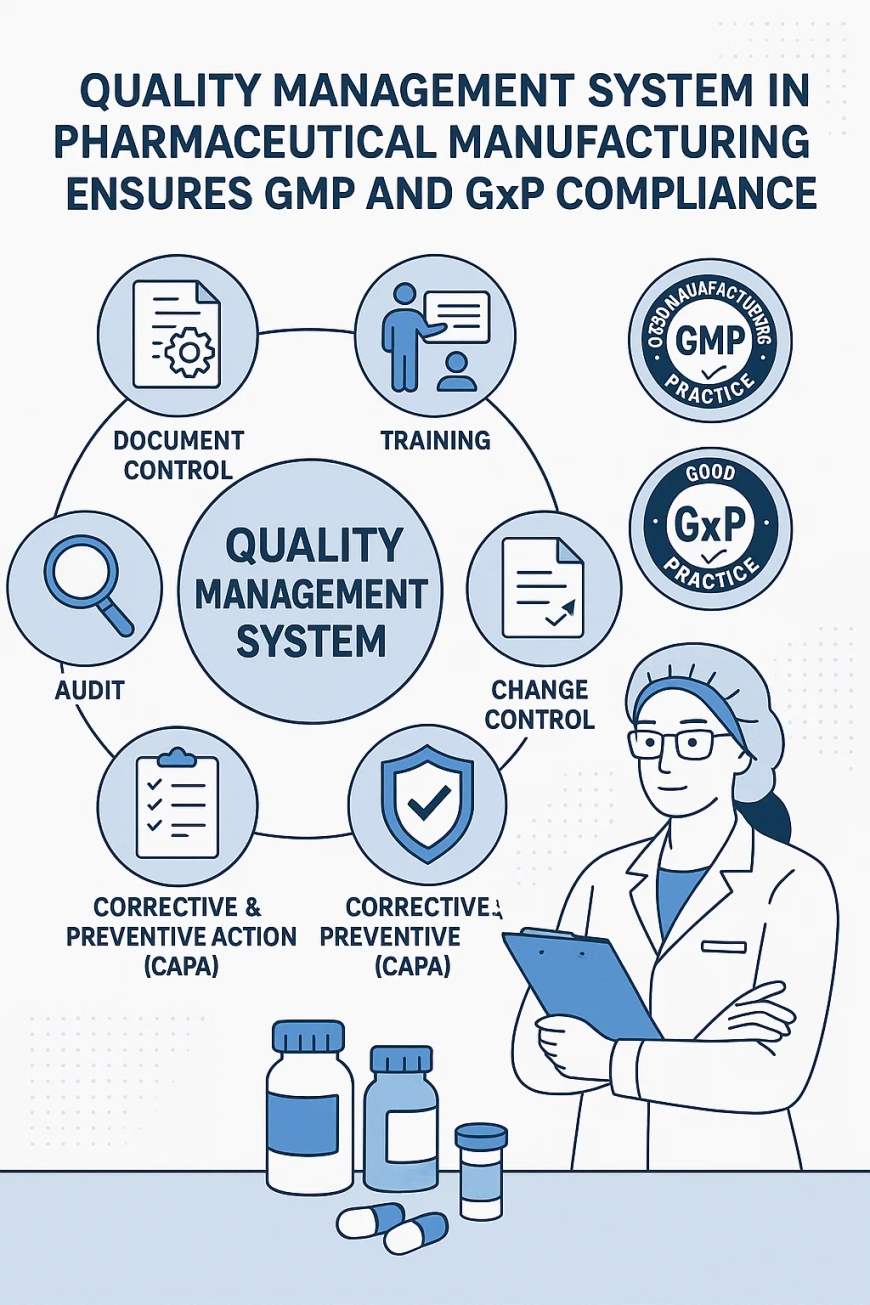
How a Quality Management System in Pharmaceutical Manufacturing Ensures GMP and GxP Compliance
Compliance extends beyond internal operations. Supplier quality management is crucial in ensuring that raw materials and outsourced components meet regulatory requirements. A QMS empowers pharmaceutical manufacturers to assess, qualify, and monitor suppliers based on predefined quality metrics. Integrated workflows allow for real-time risk assessment, documentation of supplier audits, and performance tracking, thereby ensuring that the entire supply chain is compliant with GxP and GMP standards.
Driving Global Pharmaceutical Standards with a Robust Quality Management System in Pharmaceutical Manufacturing
Pharmaceutical manufacturers operate in a highly regulated environment where global standards such as Good Manufacturing Practice (GMP) and Good Practice (GxP) requirements govern quality, safety, and efficacy. A well-designed quality management system in pharmaceutical manufacturing is central to achieving and maintaining these compliance mandates. It provides a structured framework for managing processes, data, documentation, and quality assurance protocols across every phase of the product lifecycle.
The Role of QMS in Upholding GxP and GMP Across Operations
Implementing a quality management system in pharmaceutical manufacturing enables companies to align with stringent global quality standards. GxP compliance, which includes GMP, GLP (Good Laboratory Practice), and GCP (Good Clinical Practice), requires a unified and traceable system that facilitates robust oversight and documentation. A QMS ensures that data integrity, operational consistency, and process standardization are embedded into daily workflows. It allows for early identification of deviations, real-time monitoring, and efficient resolution strategies.
Strengthening Documentation and Record-Keeping with a Quality Management System in Pharmaceutical Manufacturing
Documentation is a critical component of GMP and GxP compliance. A comprehensive quality management system in pharmaceutical manufacturing ensures that every standard operating procedure, training record, batch production record, and validation protocol is captured, version-controlled, and stored securely. With integrated QMS tools, document management becomes more efficient, enabling auditors and inspectors to access the necessary files without administrative delays. Accurate documentation drives transparency and supports regulatory inspections by agencies such as the FDA and EMA.
Building a Culture of Quality Assurance with a Unified QMS Platform
Embedding quality assurance into the fabric of pharmaceutical manufacturing requires more than periodic audits. A modern QMS promotes a culture where quality management becomes an everyday responsibility across departments. Training management, deviation handling, and change control are harmonized through the system to minimize risks and ensure adherence to GMP principles. By focusing on preventive strategies rather than reactive fixes, organizations can maintain higher product integrity and reduce quality failures.
Automating Audit Trails and Deviations Through a Quality Management System in Pharmaceutical Manufacturing
Regulatory compliance requires clear and consistent audit trails. A quality management system in pharmaceutical manufacturing streamlines the creation of automated audit logs, reducing the possibility of human error and tampering. Each system transaction, from data entry to approval workflows, is recorded, time-stamped, and validated. When deviations occur, the QMS initiates real-time alerts and corrective actions, ensuring timely documentation and resolution in alignment with GxP requirements.
Enabling Scalable Quality Assurance Through Cloud-Based QMS Systems
Pharmaceutical companies must scale their operations without compromising on quality. A cloud-enabled quality management system offers flexibility, centralized control, and global collaboration capabilities. These features are especially valuable for multinational organizations with facilities in different countries that need to maintain uniform GMP practices. A centralized QMS enables a harmonized quality assurance strategy, reducing variability and improving cross-functional visibility.
Managing Supplier Quality and Risk with QMS-Driven Workflows
Compliance extends beyond internal operations. Supplier quality management is crucial in ensuring that raw materials and outsourced components meet regulatory requirements. A QMS empowers pharmaceutical manufacturers to assess, qualify, and monitor suppliers based on predefined quality metrics. Integrated workflows allow for real-time risk assessment, documentation of supplier audits, and performance tracking, thereby ensuring that the entire supply chain is compliant with GxP and GMP standards.
Achieving Continuous Improvement Through CAPA and Quality Metrics
Continuous improvement is a cornerstone of effective quality management. A modern QMS facilitates the implementation of CAPA (Corrective and Preventive Actions) mechanisms that address root causes and prevent recurrence. It integrates tools for trending and analyzing quality metrics, enabling pharmaceutical companies to refine processes and reduce non-conformances. This proactive approach ensures that quality issues are not only resolved but are also prevented in the future, reinforcing GMP compliance.
Integrating Risk Management with the Quality Management System in Pharmaceutical Manufacturing
Risk management is essential in ensuring patient safety and product reliability. A robust QMS integrates risk-based thinking across all operational layers, from clinical trials to commercial production. Tools for risk assessment, control, and mitigation are built into the system, enabling pharmaceutical organizations to align with ICH Q9 principles and other GxP frameworks. By incorporating risk management into everyday practices, companies can anticipate quality challenges and take action before issues escalate.
Streamlining Change Control Processes and Version Management
In pharmaceutical manufacturing, change control is a regulated process that must be managed meticulously. Whether changes are made to formulations, processes, or equipment, the QMS ensures that they are properly reviewed, documented, approved, and implemented. The system tracks version history and ensures that only authorized personnel can make changes. This prevents discrepancies that could lead to compliance violations or product recalls.
Enhancing Cross-Functional Collaboration with Centralized QMS Dashboards
Todays pharmaceutical manufacturing landscape requires a unified approach to quality assurance across R&D, production, quality control, and regulatory affairs. A centralized QMS dashboard offers a single source of truth for all stakeholders, improving collaboration, communication, and alignment. It allows quality teams to monitor KPIs, audit statuses, CAPAs, and training metrics in real time, ensuring consistent performance across the enterprise.
Regulatory Readiness and Inspection Preparedness with a Quality Management System in Pharmaceutical Manufacturing
Regulatory inspections are a constant reality for pharmaceutical companies. A digital QMS ensures that every compliance requirement is documented, accessible, and verifiable. With integrated workflows for audit management, SOP adherence, and deviation tracking, companies can demonstrate control and traceability at all times. This enhances confidence during FDA and EMA inspections and minimizes the risk of citations or enforcement actions.
Conclusion: Why ComplianceQuest Is Essential for Quality-Driven Pharmaceutical Enterprises in 2025
As the pharmaceutical industry evolves to meet increasing global demand and regulatory complexity, the need for a robust and intelligent QMS becomes even more critical. ComplianceQuest stands at the forefront of this transformation by delivering a next-generation quality management system in pharmaceutical manufacturing. Designed to meet the dynamic requirements of GMP and GxP compliance, ComplianceQuest integrates advanced tools for document control, CAPA, change management, and supplier quality into a single, cloud-native platform. In 2025, organizations seeking resilience, scalability, and regulatory excellence cannot afford to rely on fragmented systems or manual processes. With ComplianceQuest, pharmaceutical leaders are empowered to build a quality-first enterprise that prioritizes compliance, innovation, and continuous improvement across every level of the business.